
If spreadsheets fire your label print jobs, vendor software runs on three PCs, and a Word document holds “approved templates” nobody follows, your labelling system is broken.
Mislabelled shipments cost hundreds to thousands in rejections.
Compliance failures trigger recalls. Staff waste hours monthly fixing preventable errors.
The difference between firms that fix labelling and those that stall isn’t budget or technology. It’s understanding which business pressures, operational realities, and regulatory constraints matter most.
This article maps those forces and shows how they dictate architecture decisions before you write a single template or configure a printer.
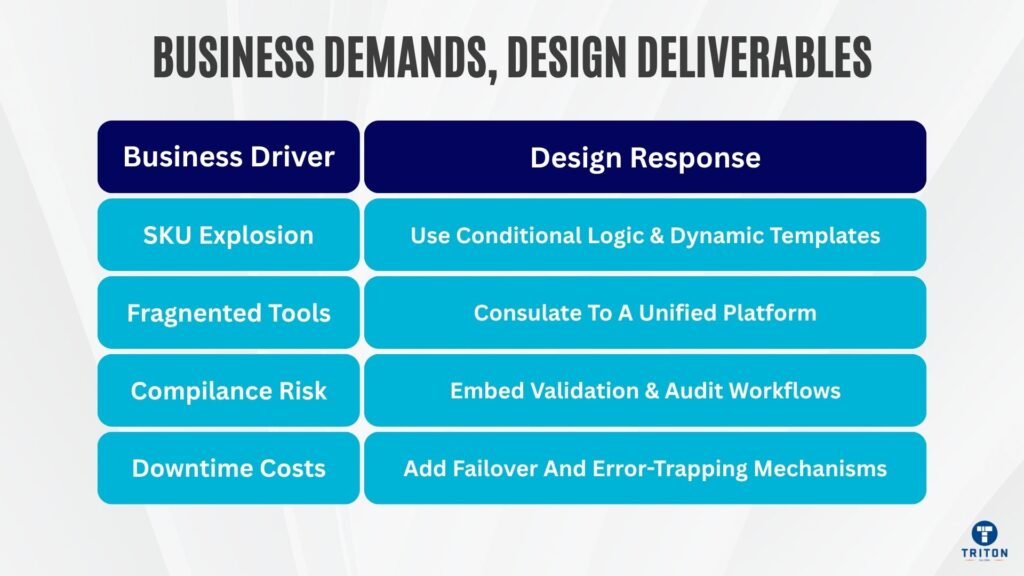
A manufacturer with 200 SKUs prints c. 2,000 label variants annually when regional rules differ. One product becomes ten label designs: different languages, compliance statements, distributor logos, or pack sizes. Without branching logic in your label templates, you duplicate and maintain variants manually. Operators select the wrong templates. Version drift occurs, and training costs multiply.
Spreadsheets fire print macros. Vendor software sits on three PCs. Scripts pile up. Organisations overspend 15-30% on IT software due to underutilisation and overlapping features. Each silo requires its own support, version control, and training. Consolidating tools cuts tool-related costs by up to 30% and improves staff productivity by 25%. Unified systems-whether BarTender-driven or another platform-strengthen consistency, auditability, and agility whilst slashing total cost of ownership.
Labels caused 45.5% of 422 US food recalls in 2024, costing the industry $1.92 billion in direct recall expenses. Each recall averages $10 million, not including lawsuits, reputational damage or lost sales. Misprints, omissions or outdated versions trigger resets, operator interventions and rework that cascades through production lines. Validation logic, error trapping and reprint policies contain these costs before they get out of control and become full recalls or shipment rejections.
Label history is your data trail. When components or finished goods must be traced retroactively, you rely on print logs, version links, and archival templates to reconstruct what was printed, when, and under which approval. Without visibility, recall spirals as you scramble to identify affected batches, audits fail because you cannot produce evidence of compliance, and brand reputation suffers permanent damage. In regulated environments, such as medical devices, chemicals, and food, this traceability isn’t optional.
Remote warehouses demand mobile printing, shipping docks need handhelds, and cloud agents feed IoT monitoring infrastructure. If your architecture is rigid today, you will reengineer tomorrow when business demands shift. Design for expansion from day one: remote agents, mobile workflows, and monitoring infrastructure that tolerates future shifts without requiring a rebuild or forcing you to run parallel systems during migration.
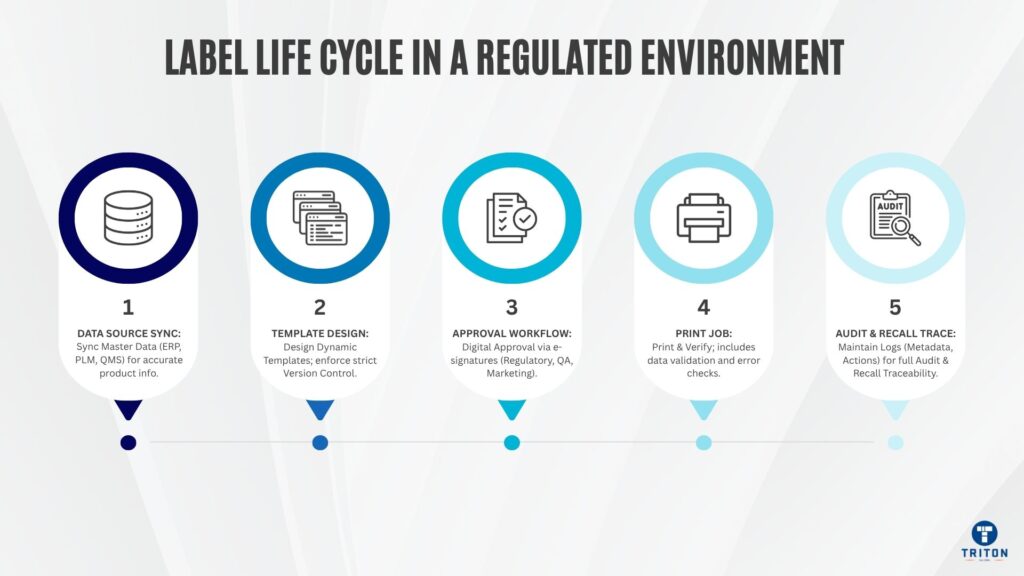
Five operational archetypes drive your design. Each demands a different architecture.
As goods are received, processed, or shipped, your systems fire a label job. The label solution must ingest transaction data, enrich it via master data lookup, resolve the correct label variant and printer, and print reliably. Latency, error handling, and concurrency determine whether your production line runs smoothly or stalls waiting for labels.
Some labels are legislated: GHS/CLP for chemicals, UDI for medical devices or local language requirements in Australia and New Zealand. Your templates must dynamically include or exclude regulatory blocks, support multilingual layouts and switch logic by region or product class. Errors here will result in shipment rejections at customs or compliance failures during regulatory inspections.
Operational realities require ad hoc labels-repair tags, internal worksheets, sample labels or deviation tags-without submitting IT tickets. The system must allow controlled, permissioned ad hoc prints and prevent operators from bypassing governance or introducing labelling errors that pollute your audit trail.
High integrity labels like UDI, serialised barcodes and compliance labels need to be verified before they hit the production line. A scanner or camera reads the printed barcode, checks it against ANSI/ISO standards and signals pass or fail. Failures trigger automatic reprints or operator alerts so defective labels don’t get into circulation.
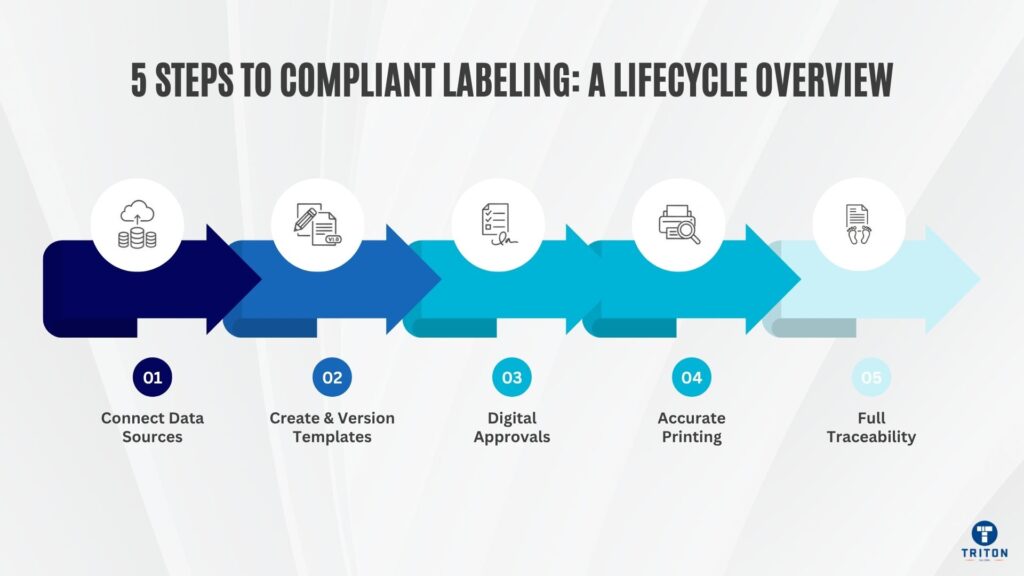
Regulatory and standards constraints are non-negotiable. Violate them, and your labels get rejected at borders, trigger recalls, or fail audits. Your system must encode these rules from day one.
Most global labelling ecosystems are built upon GS1 standards. GTIN, SSCC, GS1-128, GS1 DataMatrix, and the evolving GS1 Digital Link act as lingua franca across supply chains. If your label encoding logic or data pipelines violate GS1 rules-AI ordering, group separators, fixed/variable fields-downstream systems will reject your labels. Always code with GS1 spec alignment in mind.
If you manufacture medical devices or components destined for regulated markets, UDI is non-negotiable. In the US, the FDA mandates that devices bear a Unique Device Identifier composed of a Device Identifier (DI) and optionally Production Identifiers (lot, expiration, and manufacture date).
The DI is often implemented via GTIN, as GS1 is an FDA-accredited issuing agency.
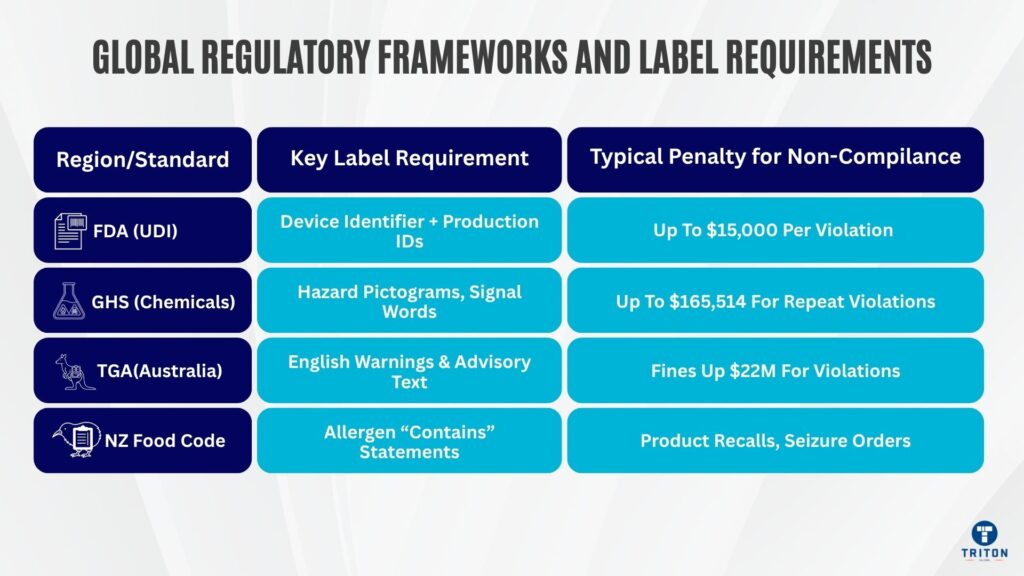
Non-compliance is classified as misbranding, with potential enforcement actions including seizure, injunction, and civil and criminal penalties. Fines run up to $15,000 per violation and up to $1,000,000 per proceeding. Rules state that if any production information appears on the label, it must be part of the UDI barcode.
UDI labelling demands immutable template versions, audit trail retention, and compliance with direct-marking rules when labelling on devices themselves. In the EU, you also need to manage the Basic UDI-DI for submissions that are not printed, but used for regulatory grouping.
Chemical labels must display pictograms, signal words like “Danger” or “Warning”, hazard statements and precautionary phrases. In 2023 there were 3,213 OSHA HazCom related violations, a 19% increase from 2022 and over $50 million in fines.
Effective January 2025, OSHA’s maximum civil penalties are $16,550 for serious violations and $165,514 for willful or repeat violations.
Regional variation across the EU, Australia, New Zealand, and the US means your template logic must conditionally include correct sets.
Australia adopted the Globally Harmonised System in 2012, requiring specific pictograms and hazard statements on workplace chemicals.
New Zealand enforces similar requirements under the Hazardous Substances and New Organisms Act. If labels cross borders local regulators may require multilingual versions or additional disclaimers.
Australia’s Therapeutic Goods Administration requires specific labelling for therapeutic goods including active ingredients, warnings and mandatory advisory statements in English. In 2024 Medtronic was fined $22 million for supplying medical devices not on the Australian Register of Therapeutic Goods, the largest fine ever under the Therapeutic Goods Act.
Advertising violations result in fines of $3,960 for individuals and $19,800 for corporations per infringement.
New Zealand’s Food Standards Code mandates allergen declarations in bold within ingredients lists, plus a separate “contains” statement summarising allergens.
Non-compliance can result in recalls, closure orders, seizure of products, criminal prosecutions, and fines.
Templates must include logic for these variations without requiring manual intervention.
In regulated industries, specific laws govern how you manage label data. In the US, 21 CFR Part 11-the FDA regulation covering electronic records and signatures-requires that template versions, user actions, print logs, and metadata be preserved for years.
ISO 13485, the international standard for medical device quality management systems, mandates similar controls.
Once a template version is approved for use, it cannot be changed retroactively. Decommissioned versions must remain auditable so you can prove what was in production at any given time.
Additionally, any print job must carry traceable metadata-user, timestamp, version, and input data, so you can reconstruct historical prints when regulators or auditors demand evidence of what was printed, when, and under whose authority.
About 70% of the total fixed cost of the label is locked in during design. Without conditional logic, localisation, variable modules, and versioning, you duplicate templates excessively.
A manufacturer selling into five regions with three pack sizes creates fifteen templates manually when one template with branching logic suffices. Each duplicate requires separate maintenance, approvals, and version tracking. When regulations change, you update fifteen templates instead of one.
Templates must connect to canonical master data. Label systems cannot operate as islands. Product specifications, compliance requirements, and regulatory text live in ERP, PLM, or quality systems.
Templates validate against these sources at print time. When product formulas change, compliance teams update master data once, and every label variant reflects changes automatically. Without this coupling, you manually synchronise changes, and errors multiply.
Versioning discipline separates working systems from disasters. Once a template prints in production it’s immutable. Auditors demand proof of what was printed when. Medtronic was fined $22 million in 2024 for supplying medical devices not registered in Australia. Template version control is your audit defence.
Integration architecture determines resilience. Gartner research says 75% of ERP projects fail. HP’s ERP migration cost $160 million in lost sales due to integration failures. Use REST APIs or database triggers instead of point scripts. REST interfaces allow retry logic, logging and easier changes. Database triggers fire label jobs when transactions complete, so labels print even if calling systems fail.
Error trapping prevents cascading failures. For serialised or high-risk labels, build automatic reject and reprint workflows. Australian manufacturers lose up to $260,000 per hour in unplanned downtime.
Verification workflows catch defects before labels leave printers. Systems scan barcodes, grade them against standards, and either release labels or trigger automatic reprints.
Scalability matters during peaks. Shipping operations burst during quarter-end or holidays. Print engines must manage queuing, load balancing, and failover without manual intervention. Design for peak load, not average load.
Build compliance controls natively. Label errors caused 45.5% of US food recalls in 2024, costing $1.92 billion. Architecture must enforce template approval workflows, change logs, access controls, and audit trails from day one.
Design for extensibility. Over 2.3 million mobile barcode printers were sold globally in 2024, up 34% from 2023. Design print agents that sync via API. Remote agents queue jobs locally, print when available, and report status to central systems.
Medium-sized businesses that address these design implications early build systems that grow with demand. Those who skip foundational decisions spend years retrofitting capabilities they should have built initially.
Your labelling system architecture determines whether compliance failures cost thousands or millions. Medium-sized manufacturers fix label chaos by aligning business drivers, operational requirements, and regulatory constraints before configuring systems.
Master data integration, REST APIs, queue management, and version control aren’t optional-they’re foundational. Firms that resolve these decisions early build systems that scale. Those who defer them retrofit for years.
Triton Store supplies industrial label printers, consumables, and verification systems for compliant labelling infrastructure.